Dielectric Barrier Discharge Treatment of Skin
1. Introduction
The application of atmospheric pressure plasmas to human tissue has been shown to have thera-peutic effects for wound healing and in treatment of skin diseases [1]. Two approaches are being followed in the use of non-thermal atmospheric pressure plasmas in medicine. In the first, the plasma is produced remotely, and its afterglow is delivered in a plume to the biological tissue. In the second approach, plasmas are generated in direct contact with living tissue. When dielectric barrier discharges (DBDs) are used for this purpose, the plasma device typically contains the powered electrode while the tissue is the counter electrode [2]. The therapeutic and sterilizing effects of plasmas, and those produced by DBDs in particular, may be attributed to several processes - production of fluxes of radicals and charged species onto cell surfaces, production of energetic fluxes of ions and photons impinging onto wounds and tissue surfaces, and generation of surface and intracellular electric fields. For example, NO which is produced in DBDs sustained in air is known to promote and regulate wound healing [3]. Electric fields of sufficient magnitude can initiate electroporation. In this project we focus on the production of large electric fields in the head of DBD filaments, the interaction of those fields with human skin cells, cell membranes and cell nuclei, and the intracellular production of electric fields through that interaction. We also address the production of fluxes of radicals and charged species onto cell surfaces.
2. Electroporation theory
The manner in which electric fields affect cells is determined by the interplay between their conductive cytoplasm and their surrounding dielectric-like cell membranes [4]. When electric fields are applied to cells, the resulting current produces the accumulation of electric charges at the less conductive cell membranes which consequently produces a voltage drop across the membrane. Conventional electroporation uses electric field pulses of tens of microseconds to milliseconds, durations that are longer than the cell membrane charging time. This produces a voltage of approximately 0.1-1 V across the membranes, which may cause structural changes and the formation of pores in the membrane. This is a useful tool in drug therapies because the pores typically reseal without directly detrimentally affecting the cell. The electric field plays the dual role of promoting pore formation and acting as a force to drive ions through the pores. The electric fields required for electroporation depend on the duration of the applied pulse [5].
3. Description of the model
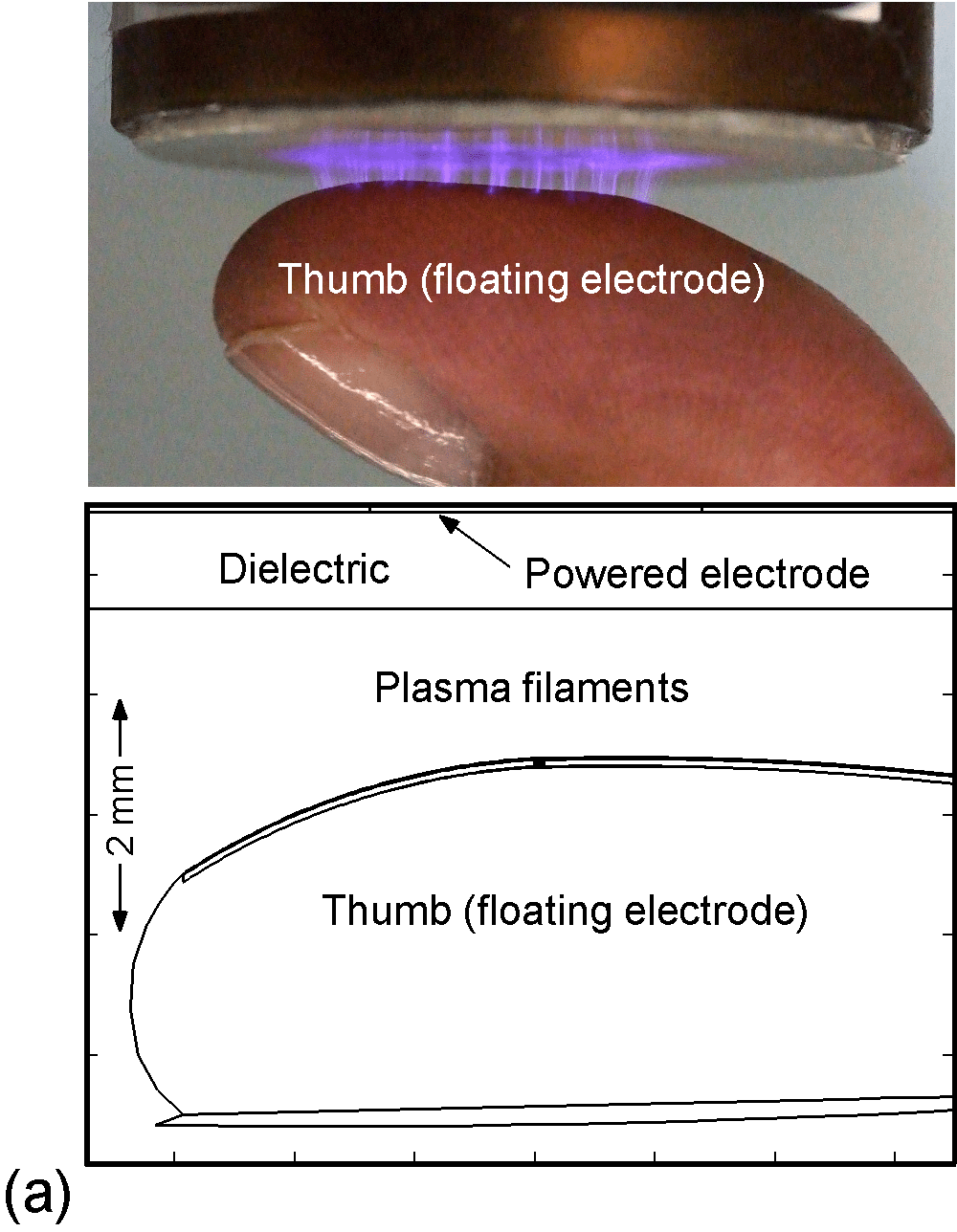
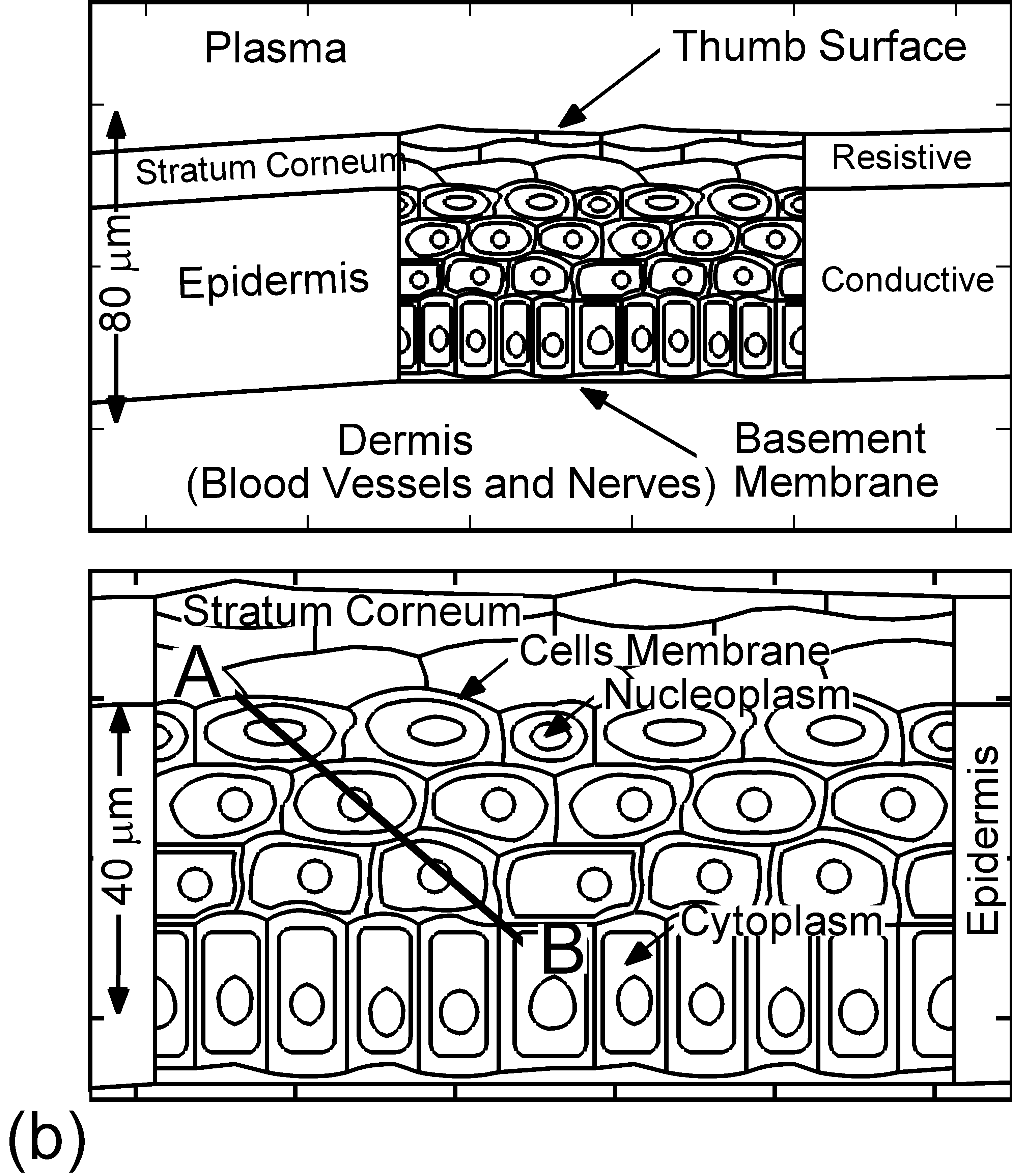
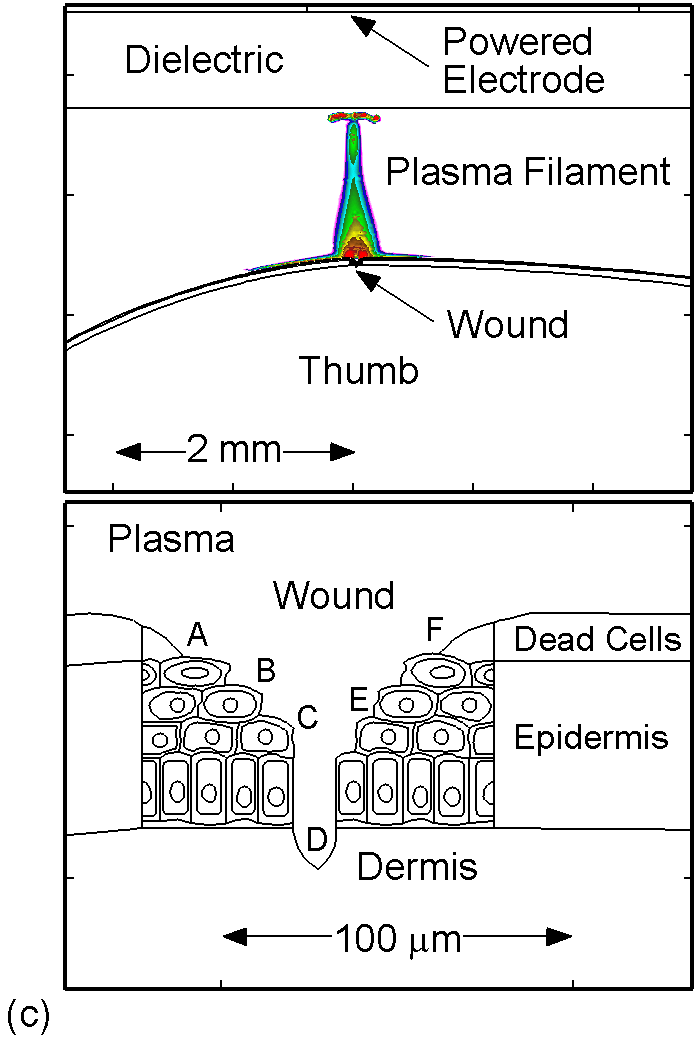
The model used in the investigation, is nonPDPSIM. nonPDPSIM is a 2-dimensional simulation in which Poisson's equation for the electric potential, and transport equations for charged and neutral species are solved. The model geometry is shown in Figure 1a [6]. A dielectric barrier discharge is positioned a few mm above a thumb having a curved surface with respect to the plasma applicator. The top boundary of the computational domain is the powered metal electrode of the DBD. The numerical grid uses an unstructured mesh with triangular elements and refinement regions to resolve the details of the plasma filaments, cell interior and nuclei. The gas mixture is atmospheric pressure humid air, N2/O2/H2O = 79/20/1. We resolved the cellular structure in a small patch approximately 100 μm wide and 80 μm deep, as shown in Figure 1b. We reproduced the epidermis layer with the layer of dead cells, a layer of horizontal and basement cells. The membranes are made thicker than in reality in order to resolve their interiors. The wound is represented as a small slice taken out of the skin down to the dermis which exposes live cells to the plasma as shown in Figure 1c.
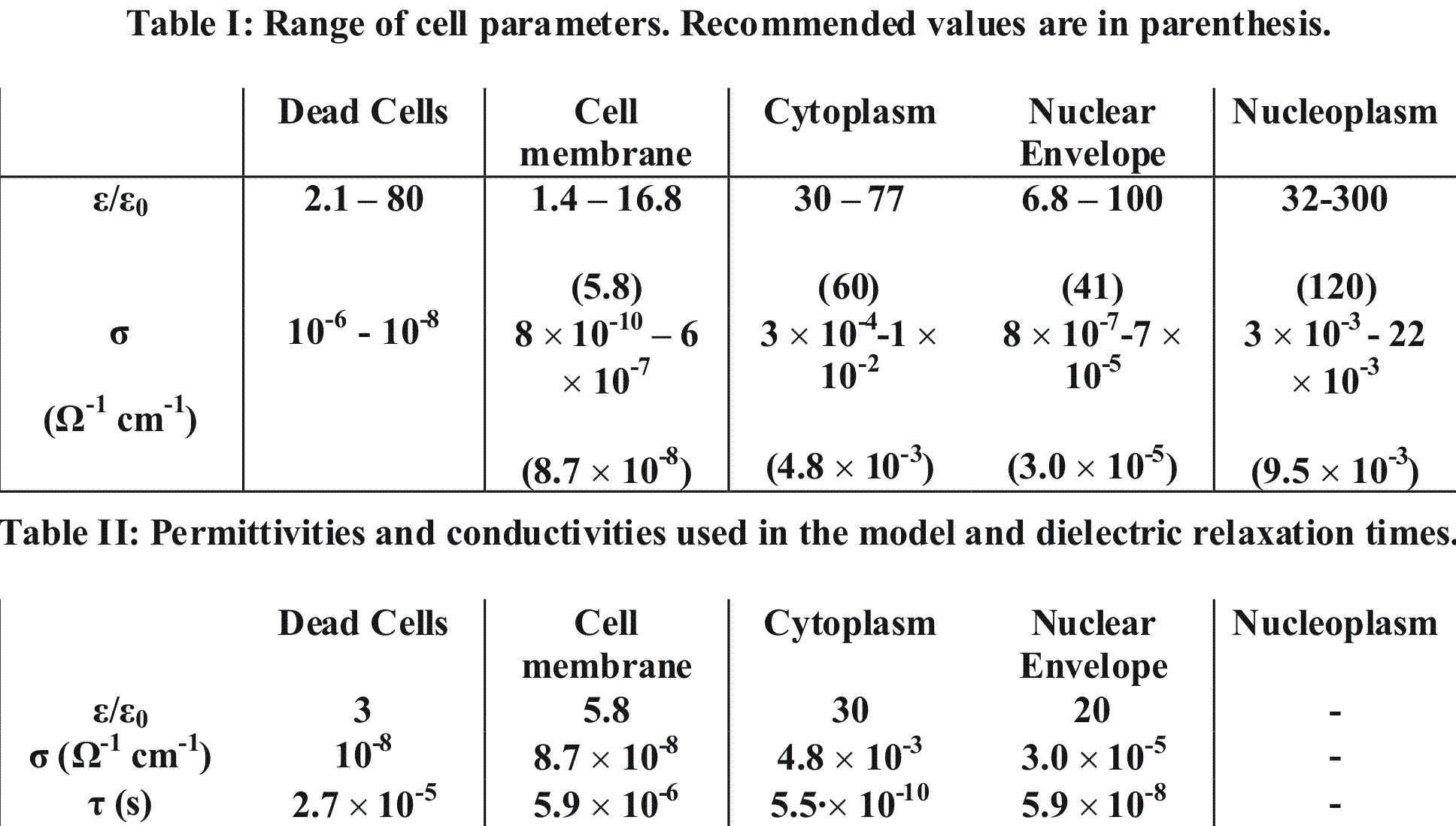
The values for dielectric constants, ε, and conductivities of cell membranes, cytoplasm, nuclear membranes and nucleoplasm were taken from [7]. The range of cell parameters are shown in Table I, where lower and upper experimental limits are shown, and recommended reference parameters are given in parenthesis. We adopted recommended values for cell parameters, except for the dielectric constants for cytoplasm and the nuclear envelope where lower limits were used.
|
|
|
Figure 1. DBD treatment of human skin. (a) Plasma applied to a thumb acting as a floating electrode in a DBD in air and schematic of the model geometry and computational domain, (b) representation of skin in the model: the stratum corneum layer which is highly resistive, layers of epidermal cells with nuclei and the dermis, (c) the wound is represented as a small slice taken out of the skin down to the dermis which exposes live cells to the plasma. |
Tables I-II: Electroporation parameters.
4. Propagation of plasma filaments and interaction with skin
In the experimental device we modeled the powered electrode is covered by a dielectric and the second electrode is a human skin or other tissue. In the model, the voltage pulse has a rise-time of 0.1 ns and amplitude of -30 kV for the base case. Initially, 5 simultaneously propagating filaments were modeled without considering the cellular structure in the mesh to investigate the variability and interaction of the filaments due to the non-uniform surface presented by the thumb. Electron density for discharge with five simultaneous filaments is shown in animated Figure 2a. The microdischarges are characterized by high electron densities (2.5 x 1013 cm-3) and there is also some evidence for electrostatic repulsion between two closely spaced negative filaments. Individual filaments compete for the available surface area of the dielectric to deposit their charge.
More detailed studies of the cellular response to the plasma filaments were performed while modeling a single filament. The maximum electric field in the streamer head prior to reaching the skin is 120-140 kV/cm, as shown in Figure 2b. When the streamer intersects the skin, the gap is closed by a conductive channel and charging of the skin is rapid. This produces electric fields of 170-200 kV on the surface of the skin. The electric fields penetrating into the skin prior to and after the arrival of the plasma produce conduction and displacement currents in the tissue. The conduction currents move charges in the cells which accumulate at the interfaces between more and less conductive regions.

|

|
Figure 2. (a) Evolution of electron density for five simultaneous filaments. (a) Electric field in a single isolated DBD filament (kV/cm). |
|
5. Intracellular charging and electric fields
The dielectric relaxation times of cellular structures, τ = ε/σ, determine the importance of the capacitive or resistive component of the membrane and cytoplasm with respect to the duration of a voltage pulse. For pulse durations long compared to τ, the resistive component dominates. For pulse durations short compared to τ, the capacitive component dominates. The duration of the DBD discharge pulse and its major interaction with the tissue is a few ns, which is short compared to τ of the membrane (5.9 μs), and comparable to that of the cytoplasm (0.5 ns).
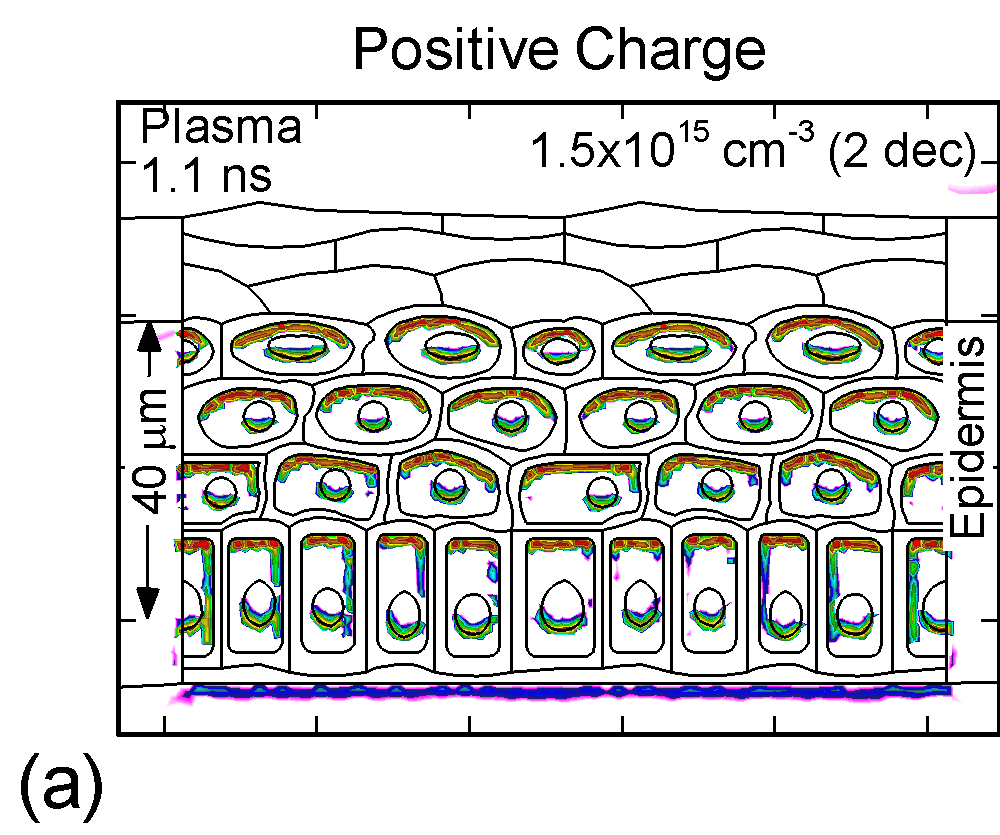
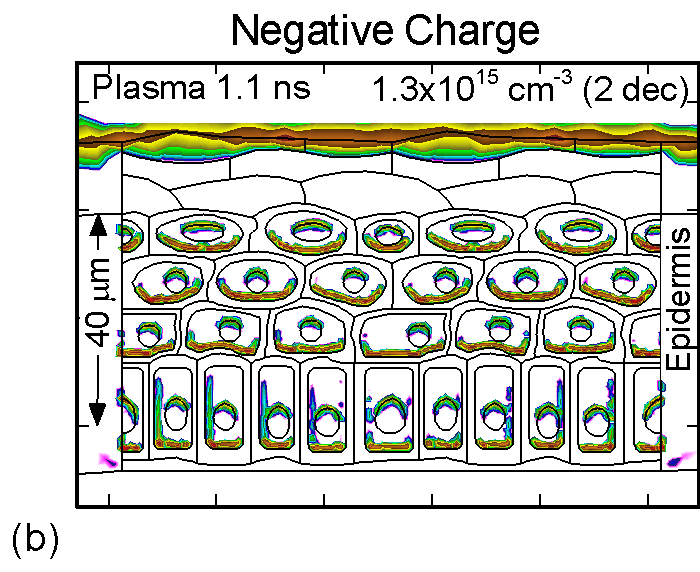
The accumulation of positive and negative charges on cell membranes and nuclei are shown in Figure 3 for the time when the gap is closed by the plasma channel and the surface of the skin charges. Positive and negative charges accumulate on opposite sides of cells membranes and nuclei. The maximum charge densities are approximately 1015 cm-3 or 1-2 x 10-4 C/cm3. The charge buildup across cell membranes required to produce potential differences of up to a few volts.
|
|
|
|
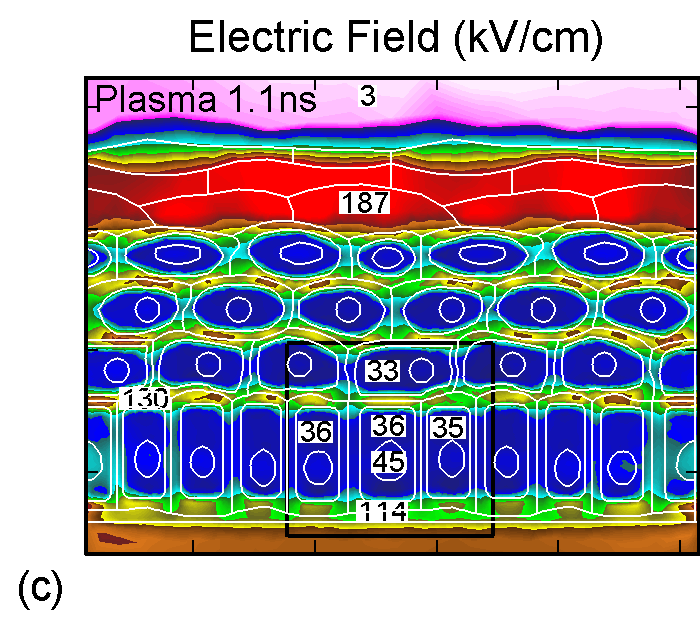
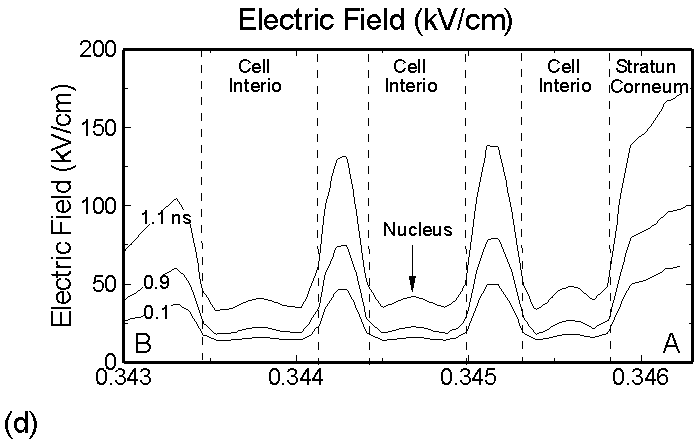
Figure 3. (a) Positive and (b) negative charge accumulation on cell membranes and nuclei, (c) electric fields inside the epidermal layer at a time when the filament strikes the surface. The largest potential drop and, as a result, the highest electric field is in the resistive stratum corneum layer. (d) Electric fields along the chord AB produced by displacement currents from the filament (0.1 ns), when the filament approaches the surface (0.9 ns) and when the filament interacts with the surface (1.1 ns). The electric field is highest (150 kV/cm) when the filament touches the thumb surface. |
|
The corresponding electric fields are shown in Figure 3c. The largest potential drop across a cell structure and, as a result, the highest electric field occurs in the Stratum Corneum layer as this layer is the most resistive. Note also the high values of electric field in fairly conductive parts of the cell–cell cytoplasm, on the order of 35 kV/cm. This indicates that electric fields produced by filamentary DBDs can penetrate the cells. Electric fields are shown in Figure 3d for a chord AB (see Figure 1b) cutting across a number of cells. The electric fields across the membrane are highest (150 kV/cm) when the filament touches the thumb surface (1.1 ns). The voltage drop recalculated for the thickness of an actual lipid membrane (5-10 nm) is about 0.1 V, a value corresponding to the lower limit of cell electroporation for long pulses.
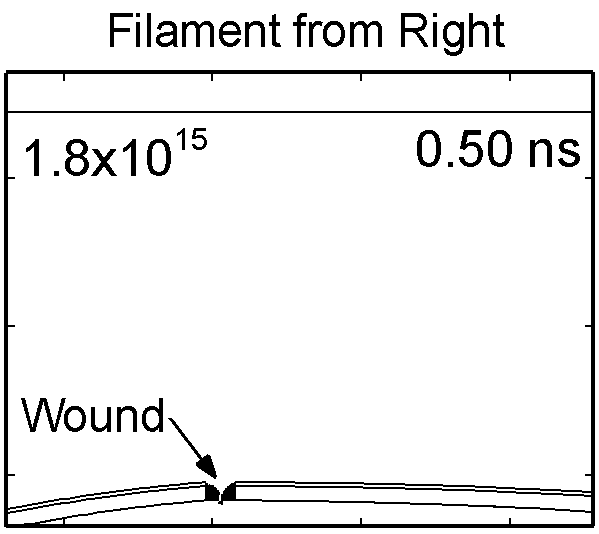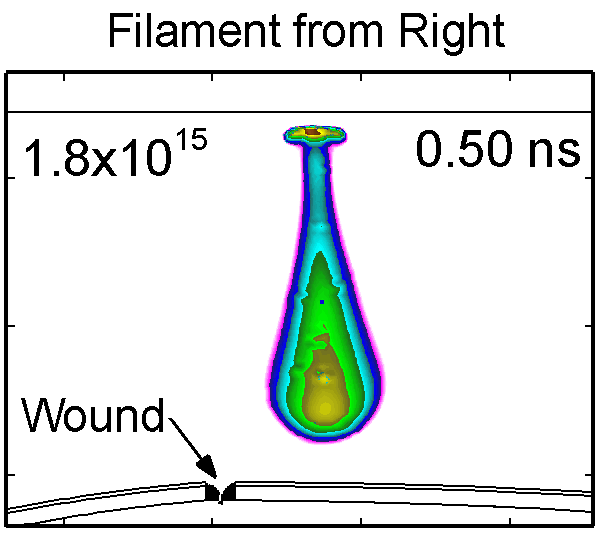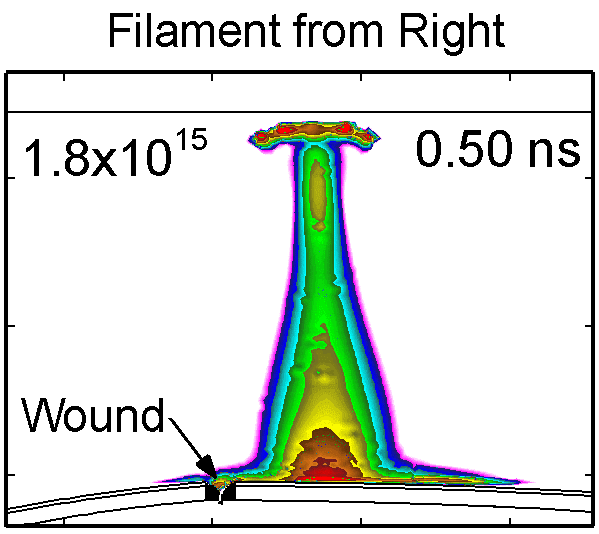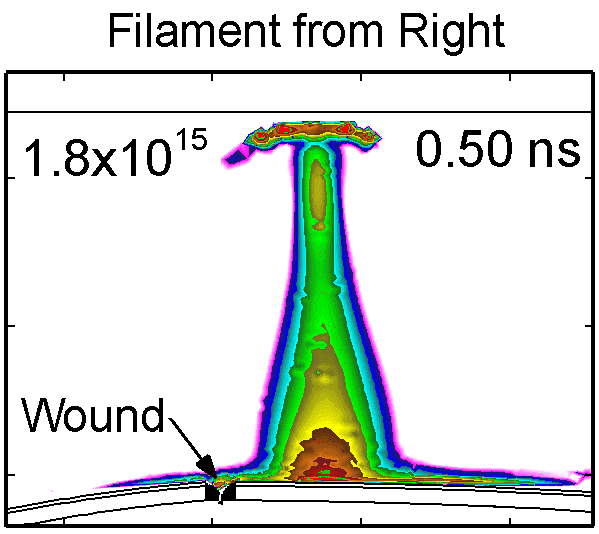
6. Dynamic of DBD over wounded skin
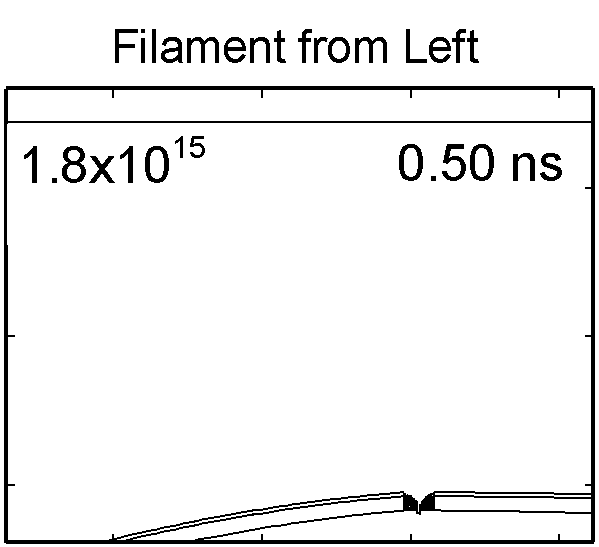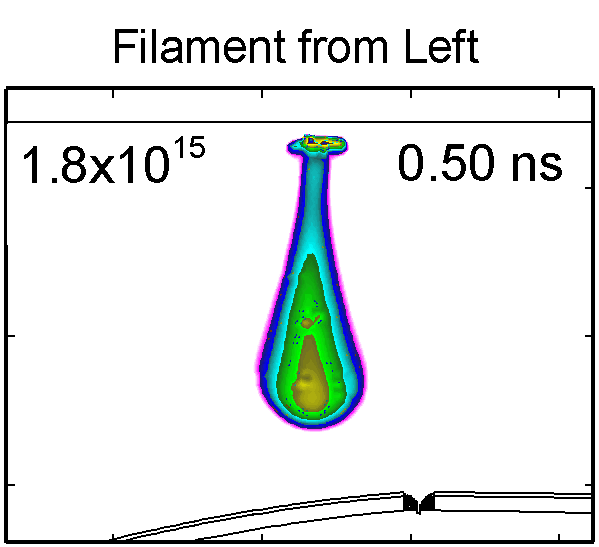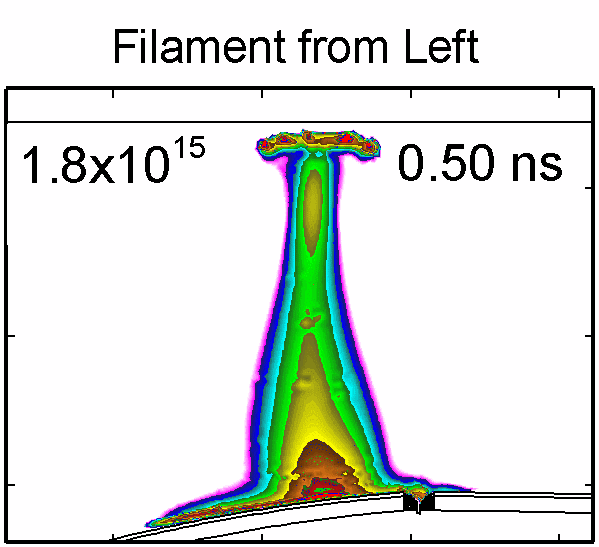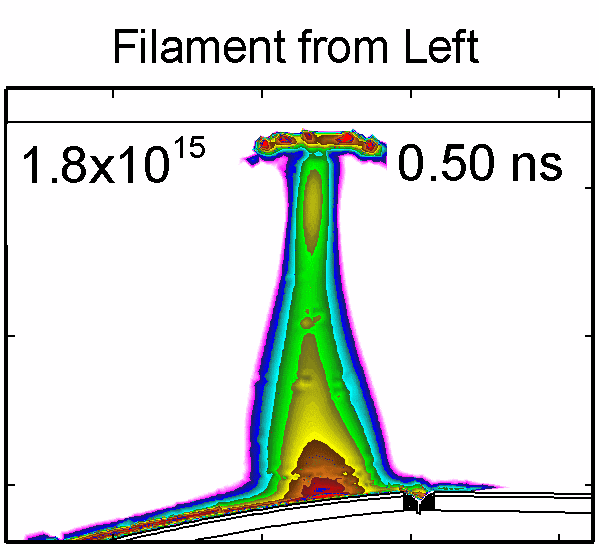
|
|
|
Figure 4. Electron density inside a bubble 900 μm in radius immersed in a dielectric liquid with (a) ε/ε0 = 2 and (b) with ε/ε0 = 80. In low ε/ε0 liquids, the streamer develops as a single filament propagating along the axis. In the high ε/ε0 liquid, the streamer propagates along the bubble surface. |
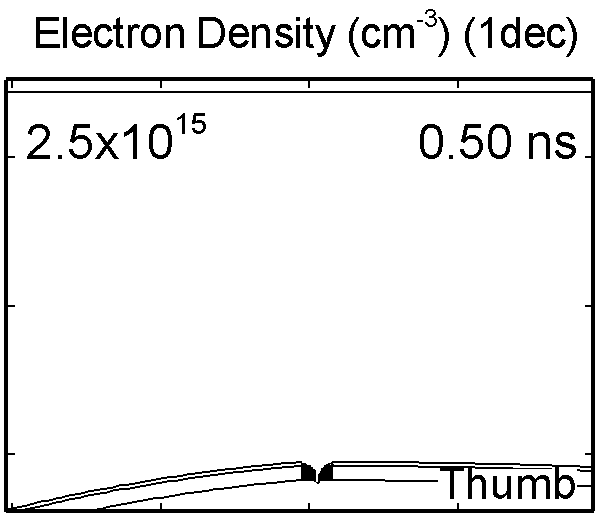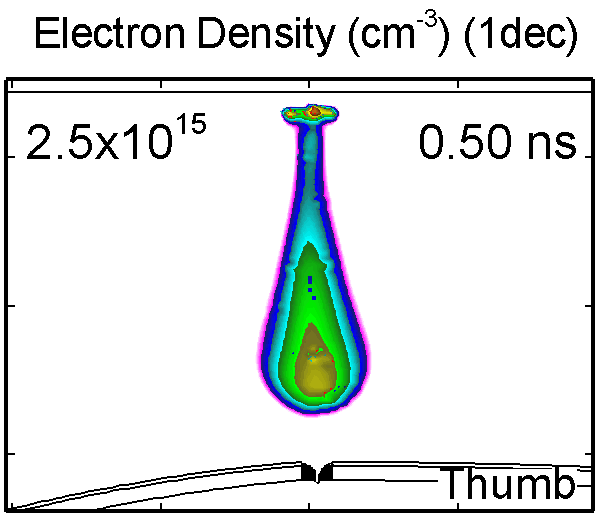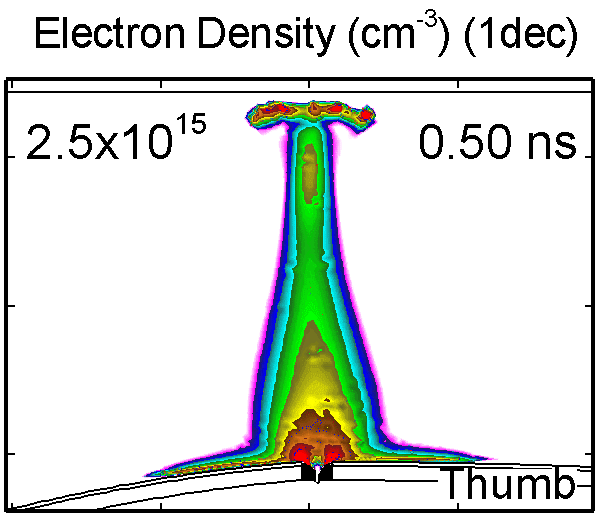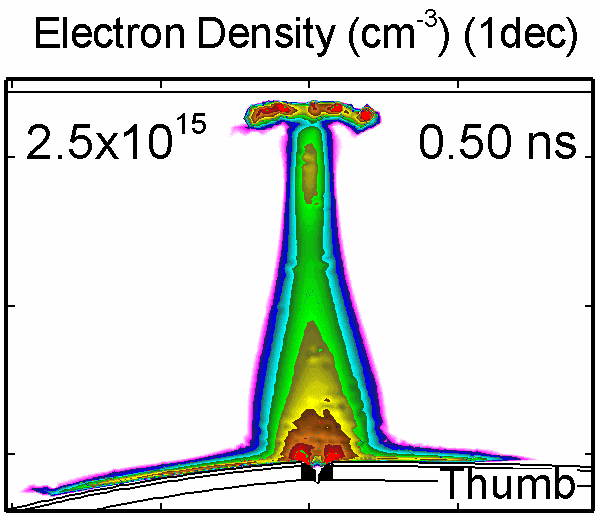
To model the dynamics of a DBD discharge over the wounded skin we used the geometry shown in Fig-ure 1c. The wound is a small slice in the skin to the dermis, which exposes live cells to the plasma. This type of wound is dry and shallow and may represent later stages of wound healing. Filaments are launched from the dielectric as shown in animated Figure 4 to intersect to the left, directly over and to the right of the wound. The skin is initially uncharged and the applied voltage is -30 kV. Upon intersection with the skin, the filaments charge the surface, producing lateral electric fields which spreads the filament over the tissue to many times its diameter. Electron densities reach 1015 cm-3, increasing near the surface due to the large capacitance of the exposed features. The Debye length of a few μm is comparable to the dimensions of the wound which enables penetration of the plasma into the wound as shown in Figure 5. As the filaments on either side of the wound spread along and charge the surface, they cross over into the wound and asymmetrically charge the exposed cells. This results in the charged particle densities in con-tact with the cells to be highest opposite the entry point of the plasma. When the filament is directly over the wound, the plasma is symmetric but concentrated near the top of the wound where the view angle to the plasma is largest. Electron impact produces radicals in direct contact with the cells, such as O and NO, shown in Figure 5. At this short time, the locations of the radicals reflect the location of their crea-tion by either electron impact (O) or neutral reactions of radicals with the feedstock gases (NO). The lo-cations also reflect the track of the streamer as it spreads along the surface and enters the wound. During the time tens between pulses, diffusion will spatially homogenize these initial densities. As such, the largest densities of radicals such as O inside the wound are these initial values.
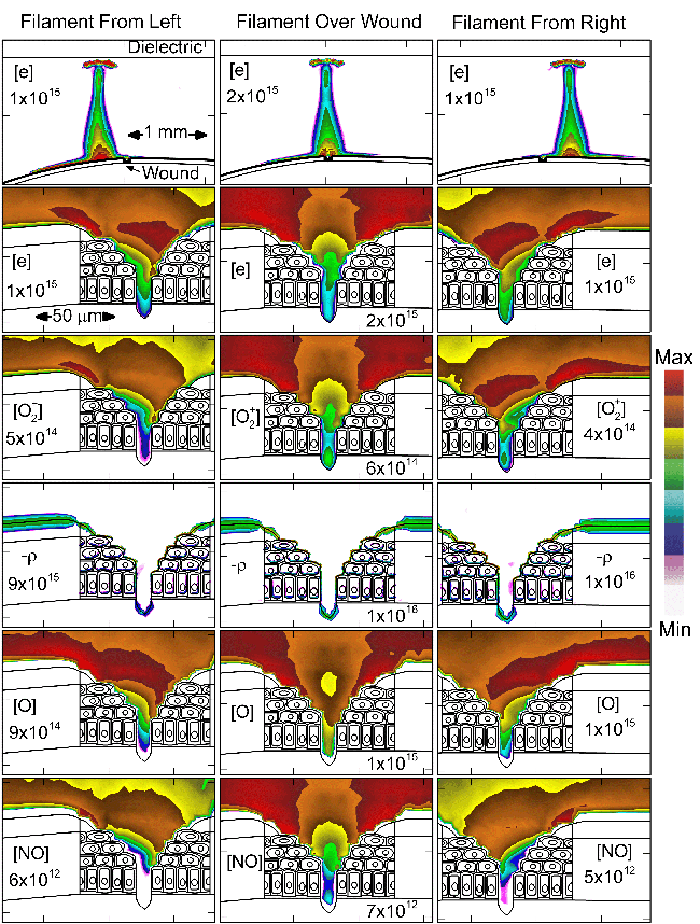 |
Figure 5. Electron density and positive charge inside a bubble 900 μm in radius immersed in a dielectric liquid with different ε/ε0. Typically, two streamers develop inside the bubble - along the axis and along the surface. The final pattern depends on ε/ε0, with higher values favoring the surface discharge. |
7. Concluding remarks
We showed that there can be significant electric field penetration into intracellular structures. Trans-membrane potentials in excess of 0.1 V, approaching the regime of electroporation, can be produced for conditions typically used for DBD plasma treatment of tissue. The potential use of DBDs for electric field therapy or electroporation will in part be determined by reproducibility. Processes such as electroporation may result from ensemble averages of the contributions of many individual filaments. The penetration of charge and neutral species into the wound is limited only by the Debye length and charging of wound's surface.
Acknowledgements
This work was supported by the US Department of Energy Office of Fusion Energy Science Contract DE-SC0001939.
References
- M. G. Kong, G. Kroesen, G. Morfill, T. Nosenko, T. Shimizu, J. van Dijk, and J. L. Zimmermann, "Plasma medicine: an introductory review", New Journal of Physics 11, 115012 (2009).
- G. Fridman,M. Peddinghaus, H. Ayan, A. Fridman, M. Balasubramanian, A. Gutsol, A. Brooks, and G. Friedman, "Blood coagulation and living tissue sterilization by floating electrode dielec-tric barrier discharge in air," Plasma Chem. Plasma Process., 26, 425 (2006).
- A. B. Shekhter, V. A. Serezhenkov, T. G. Rudenko, A. V. Pekshev and A. F. Vanin, "Beneficial effect of gaseous nitric oxide on the healing of skin wounds", Nitric oxide: Biol. Chem. 12, 210 (2005).
- J. C. Weaver, Electroporation of Cells and Tissues. (Boca Raton, FL: CRC Press and IEEE Press, 2000).
- S. J. Beebe, P. M. Fox, L. J. Rec, K. Somers, R. H. Stark, and K. H. Schoenbach, "Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition," IEEE Trans. Plasma Sci., 30, 286 (2002).
- N. Yu. Babaeva and Mark J. Kushner, "Intracellular electric fields produced by dielectric barrier discharge treatment of skin", J. Phys. D 43, 185206 (2010).
- Y. Feldman, I. Ermolina, and Y. Hayashi, "Time Domain Dielectric Spectroscopy Study of Bio-logical Systems", IEEE Trans. Dielectr. Electr. Ins. 10, 728 (2003).



